Here we come 2023!
Sold at over 500 Whole food stores, Online at Amazon and Walmart, Xynase is quickly becoming a trusted brand for nasal dryness.


Generic to Arestin Microspheres by Bausch Health (Orapharma) receives Competitive Generic Therapy De
https://www.prnewswire.com/news-releases/fda-grants-profounda-inc-competitive-generic-therapy-cgt-designation-for-planned-anda-filing-of-...


DOG AND HUMANS GET GREAT RESULTS
https://youtu.be/5KK0HYPK57c


Sales exceed $2 Million for first year of Profounda's Commercial Presence in the USA
Sales of Impavido, Rhinase Soothing Nasal Gel and Rhinase Lubricating Mist exceeded $2.5 M for 2016 making it a great start for Profounda...


Orphan drug status approval for Acanthamoeba Keratitis received by Profounda for Miltefosine
Orphan indication received by Profounda is for the treatment of Acanthamoeba Keratitis, also commonly known as AK usually associated with...


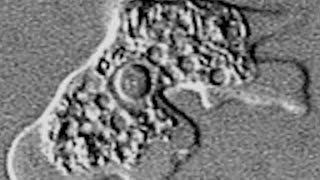
Profounda Inc. receives FDA orphan-drug designation for the Treatment of Primary Amebic Meningoencep
Orphan indication received by Profounda is for the treatment of PAM, also commonly known as the Brain Eating Amoeba ORLANDO, Fla. Dec ...
Patient survives Brain Eating Amoeba
http://www.clickorlando.com/news/broward-teen-survives-brain-eating-amoeba-was-treated-in-orlando


Profounda Licensed in Florida
Profounda has received the necessary licensure from the State of Florida as a Drug Manufacturer which allows it to supply drug directly...